Dysport® is FDA-approved for the treatment of frown lines. Off-label, it is used to treat areas that BOTOX is used for.
A popular choice and alternative to Botox®
Dysport® (pronounced Diss-port) was first launched in the UK in 1991 and has been available in the United States for cosmetic use since 2009. It smoothens wrinkles by temporarily reducing movement in certain facial muscles. It offers quick results and lasts up to four months, giving a natural and smooth look.
Many patients are convinced that Dysport works more quickly than Botox does (about two to three days compared to three to five). If you find that one product is no longer working the way you want it to, switching to one of the others may help.
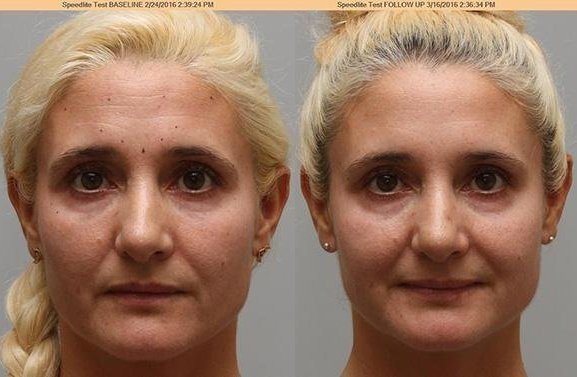
These before and after photos were taken 3 weeks apart with the patient in repose.
How are DYSPORT® and BOTOX® Cosmetic similar?
Dysport and Botox are what we call neuromodulators. They are both derived from the same botulinum toxin type A and are approved by the U.S. Food & Drug Administration (FDA). They are each manufactured by different pharmaceutical companies, and compete in both the therapeutic and cosmetic market. Many providers use them interchangeably.

Photos taken 3 weeks apart with patient actively frowning and squinting.
How are DYSPORT® and BOTOX® Cosmetic different?
At recommended doses for the temporary improvement of frown lines, a BOTOX treatment contains 0.18 nanograms of “core active neurotoxin” whereas DYSPORT has 0.27 nanograms. This might be why many patients feel that DYSPORT works more quickly than BOTOX does. DYSPORT activity spreads out more from the point of injection in comparison to BOTOX. An experienced injector selects his/her preferred “tool for the job.”





